24. Stringer, Blake; Schmeltzer, Alexandra; Ryu, C. Hyun; Ren, Hang; Luo, Long.*; Resistive Pulse Analysis of Chiral Amino Acids Utilizing Metal-Amino Acid Crystallization Differences, submitted
23. Al-Mualem, Z.; Lorenz-Ochoa, K.; Pan, L.; Ren, H.; Baiz, C.*; Controlling Hydrogen-Bonding at a Gold Electrode Interface: The Effect of Organic Cosolvents. J. Phys. Chem. Lett. 2024 Accepted
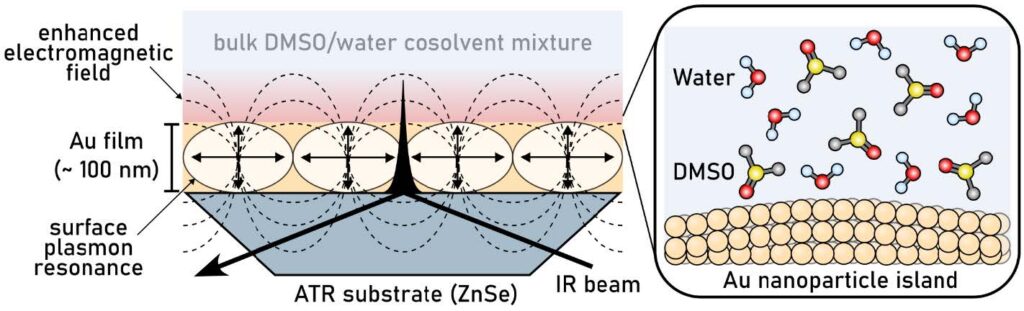
22. Lee, H.; Ren, H.*; Tuning Electrocatalytic Oxygen Reduction Reaction with Dynamic Control of Electrochemical Interfaces, J. Am. Chem. Soc., 2024, accepted 10.1021/jacs.3c13694

21. Lee, H.; Matthews, K. C.; Zhan, X.; Warner, J. H.; Ren H.*; “Precision Synthesis of Bimetallic Nanoparticles via Nanofluidics in Nanopipets”, ACS Nano, 2023, 17, 22, 22499–22507. DOI: 10.1039/D3SC01857A
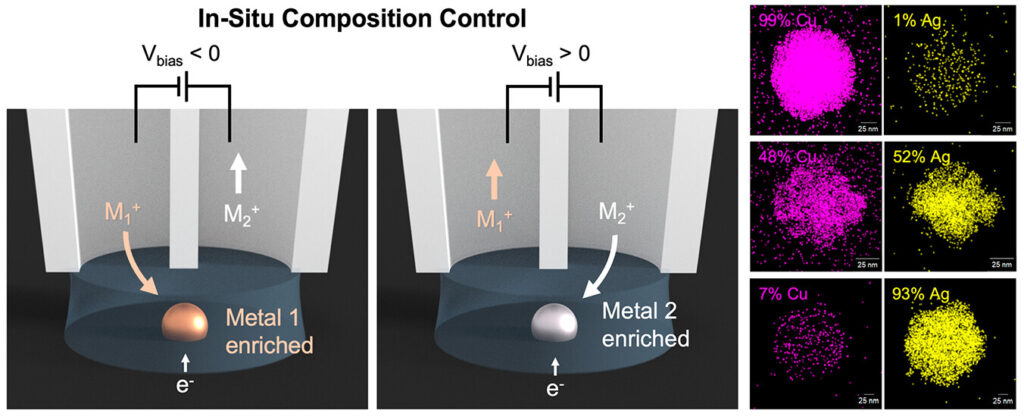
20. Mondaca-Medina, E.; García Carrillo, R.; Lee, H; Wang, Y.; Zhang, He; Ren, H. *; “Nanoelectrochemistry in Electrochemical Phase Transition Reactions”, Chem. Sci., 2023, accepted. DOI: 10.1039/D3SC01857A
19. Al-Zubeidi, A.; Wang Y.; Lin, J; Flatebo, C.; Landes, C.; Ren, H. *; Link, S.* “d-band Holes React at the Tips of Gold Nanorods”, J. Phys. Chem. Lett. 2023, 14, 23, 5297–5304 DOI: 10.1021/acs.jpclett.3c00997
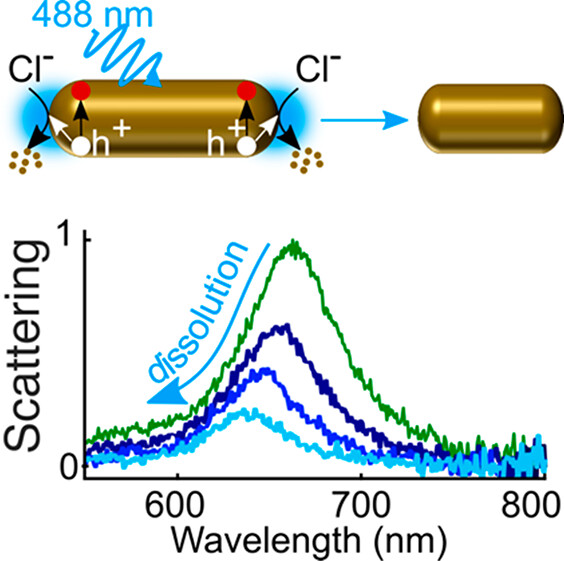
18. Wang, Y.; Li, M.; Ren, H.*, Interfacial Structure and Energy Determine the Heterogeneity in the Electrochemical Metal Dissolution Activity at Grain Boundary, Chem. Matter., 2023, DOI: 10.1021/acs.chemmater.3c00220
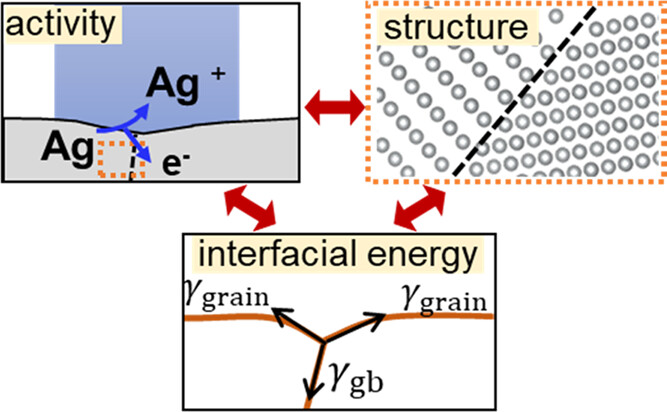
17. Ryu, C.H.; Lee, H.; Lee, H.; Ren, H.*, Learning from the Heterogeneity at Electrochemical Interfaces, J. Phys. Chem. Lett., 2022, 13, 33, 7838–7846. DOI: 10.1021/acs.jpclett.2c02009 (Perspective)
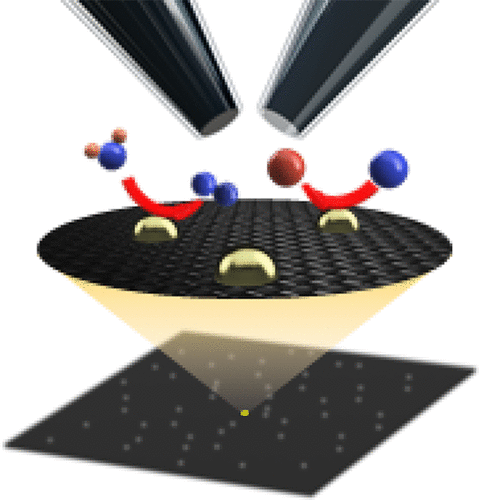
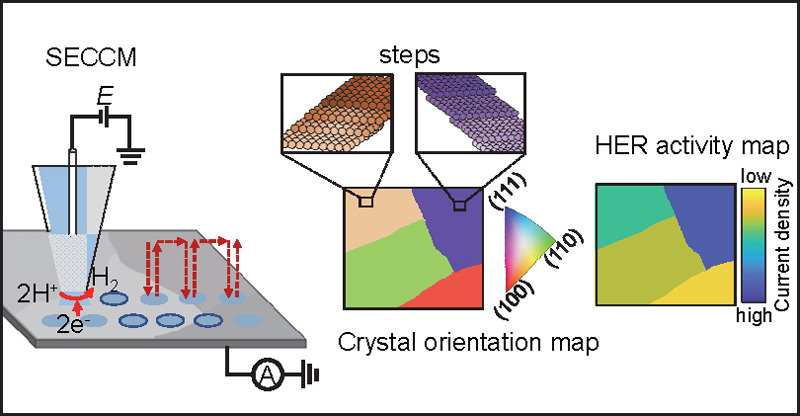
16. Wang, Y.; Li, M.; Gordon, E., Ren, H.*, Mapping the Kinetics of Hydrogen Evolution Reaction on Ag via Pseudo-Single-Crystal Scanning Electrochemical Cell Microscopy, Chinese J. Catal. 2022 43 (12), 2022 3170-3176. DOI: 10.1016/S1872-2067(22)64158-5
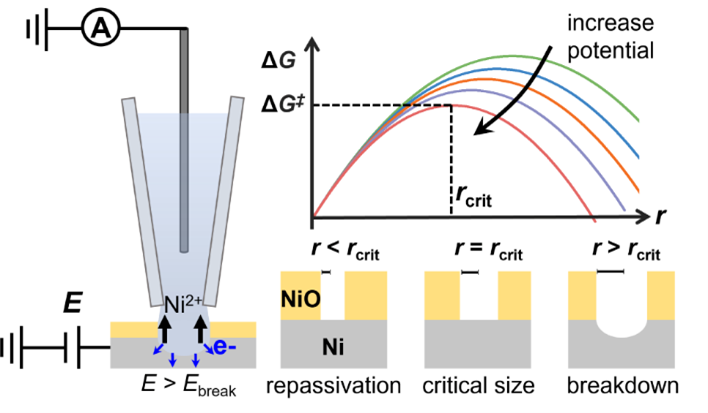
15. Li, M.; Wang, Y.; Blount, B.; Gordon, E.; Muñoz-Castañeda, J. A.; Ye, Z.; Ren, H., Stochastic Local Breakdown of Oxide Film on Ni from Identical-Location Imaging: One Single Site at a Time. Nano Lett. 2022, 22, 15, 6313–6319. DOI:10.1021/acs.nanolett.2c02018
14. Wang, Y; Li, M.; Gordon, E.; Ye, Z.; Ren, H.*, Nanoscale Colocalized Electrochemical and Structural Mapping of Metal Dissolution Reaction, Anal. Chem. 2022, 94, 25, 9058–9064. DOI: 10.1021/acs.analchem.2c01283

13. Wang, Y.; Li, M.; Ren, H., Voltammetric Mapping of Hydrogen Evolution Reaction on Pt Locally via Scanning Electrochemical Cell Microscopy. ACS Measurement Science Au, 2022 2, 4, 304–308. DOI: 10.1021/acsmeasuresciau.2c00012
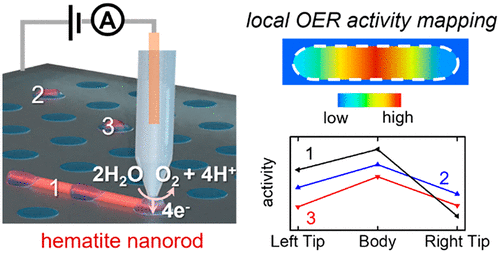
12. Li, M.; Ye, K.-H.; Qiu, W.; Wang, Y.; Ren, H.*, Heterogeneity between and within Single Hematite Nanorods as Electrocatalysts for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2022, 144 (12), 5247–5252. DOI: 10.1021/jacs.2c00506
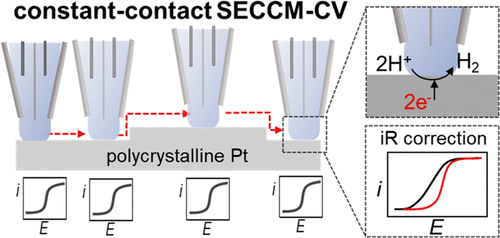
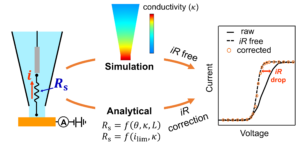
11. Blount, B.; Juarez, G.; Wang, Y.; Ren, H.*, iR Drop in Scanning Electrochemical Cell Microscopy. Faraday Discuss., 2022, 233, 149-162. DOI: 10.1039/D1FD00046B
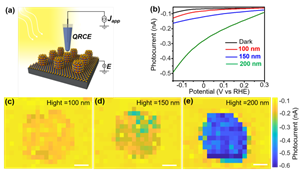
10. Zheng, H.; Li, M.; Chen, J.; Quan, A.; Ye, K.; Ren, H.; Hu, S.; Cao, Y., Strain tuned efficient heterostructure photoelectrodes. Chin. Chem. Lett. 2022, 33 (3), 1450-1454. DOI:10.1016/j.cclet.2021.08.062
9. Ren, H.*; Edwards, M. A.*, Stochasticity in Single-Entity Electrochemistry. Curr. Opin. Electrochem. 2021, 25, 100632. DOI: 10.1016/j.coelec.2020.08.014
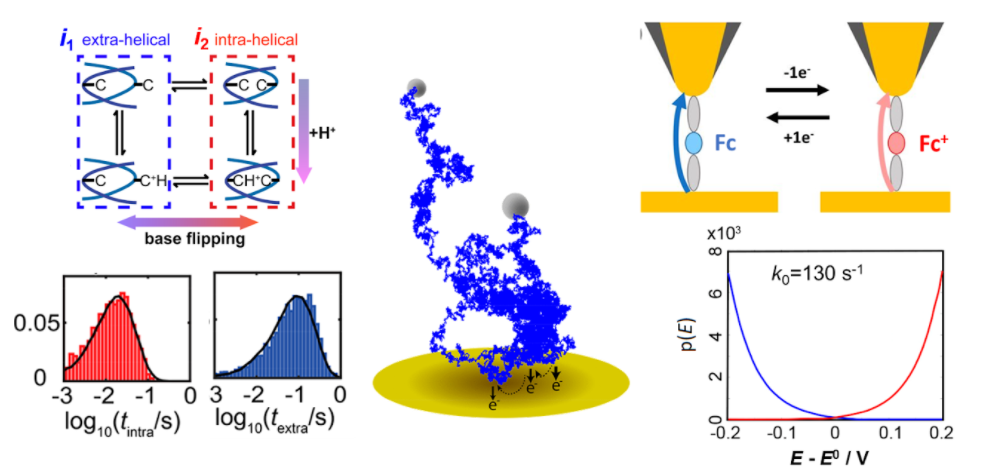
8. Liu, Y.; Jin, C.; Liu, Y.; Ruiz, K. H.; Ren, H.; Fan, Y.; White, H. S.; Chen, Q., Visualization and Quantification of Electrochemical H2 Bubble Nucleation at Pt, Au, and MoS2 Substrates. ACS Sensors 2021, 6 (2), 355–363. DOI: 10.1021/acssensors.0c00913
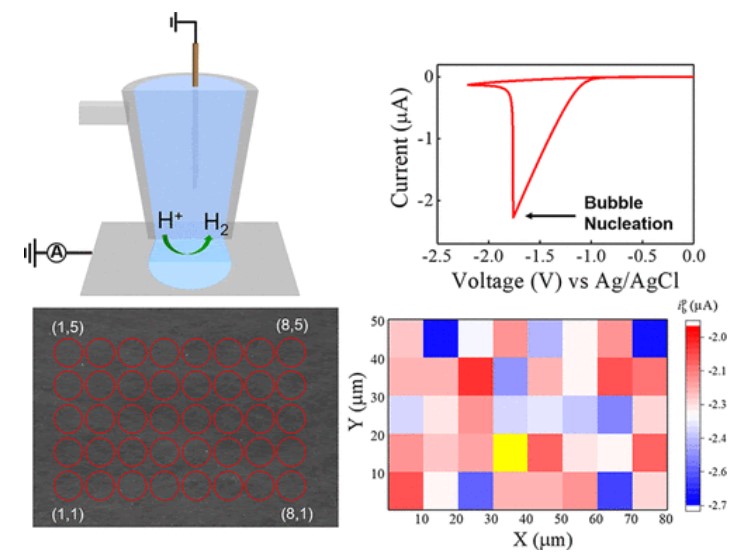
7. Allegrezza, M. L.; Watuthanthrige, N. D. A.; Wang, Y.; Garcia, G. A.; Ren, H.; Konkolewicz, D.;* “Substituent Effects in Iniferter Photo-Polymerization. Can Bond Homolysis Be Enhanced by Electronics?” Polymer Chemistry, 2020 11, 6129-6133. DOI: 10.1039/D0PY01086C
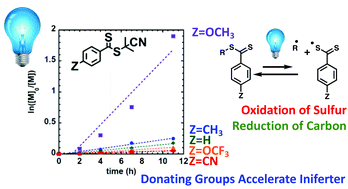
6. Blount, B.; Kilner, K.; Hu, H.; Gohmann, D.; Gordon, E.; Wang, Y.; Ren, H.*, ”Electrochemically Induced Nucleation of a Nanoscopic Ionic Solid”. J. Phys. Chem. C. 2020 124 (31), 17413-17417. DOI: 10.1021/acs.jpcc.0c05009
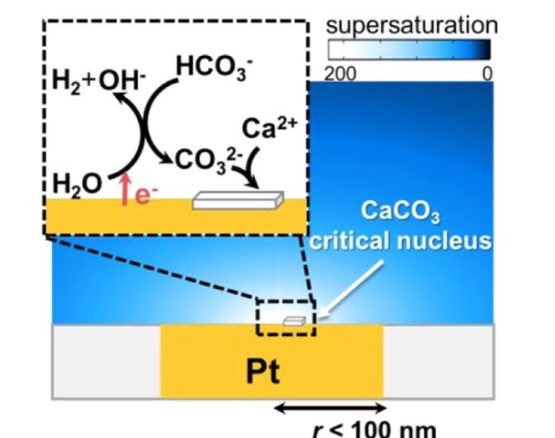
5. Ying, Y.-L.; Wang, J.; Leach, A. R.; Jiang, Y.; Gao, R.; Xu, C.; Edwards, M.; Pendergast, A. D.; Ren, H.; Weatherly Connor, K. T.; Wang, W.; Actis, P.; Mao, L.; White, H. S.; Long, Y.-T., Single-Entity Electrochemistry at Confined Sensing Interfaces. SCIENCE CHINA Chemistry 2020 63 (5), 589–618. DOI: 10.1007/s11426-020-9716-2
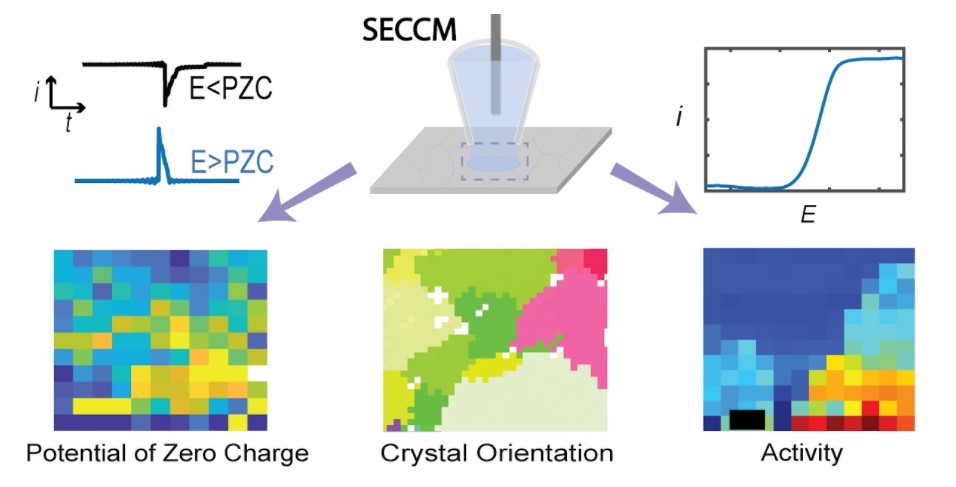
4. Wang, Y.; Gordon, E.; Ren, H.*, “Mapping the Potential of Zero Charge (PZC) and Electrocatalytic Activity of Metal-Electrolyte Interface via a Grain-by-Grain Approach”. Anal. Chem. 2020, 92 (3), 2859-2865. DOI: 10.1021/acs.analchem.9b05502
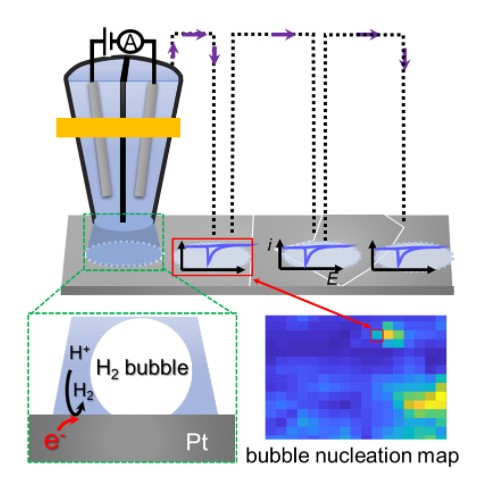
3. Wang, Y.; Gordon, E.; Ren, H.*, “Mapping the Nucleation of H2 Bubbles on Polycrystalline Pt via Scanning Electrochemical Cell Microscopy”. J. Phys. Chem. Lett. 2019, 3887-3892. DOI: 10.1021/acs.jpclett.9b01414
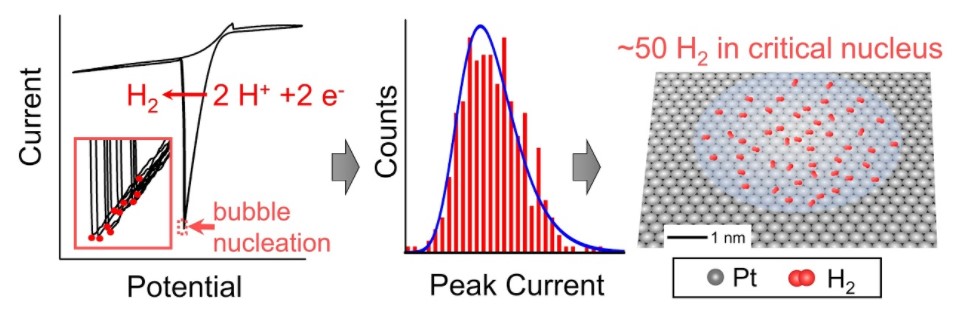
2. Edwards, M. A.*; White, H. S.; Ren, H.*, “Voltammetric Determination of the Stochastic Formation Rate and Geometry of Individual H2, N2 and O2 Bubble Nuclei.” ACS Nano 2019 13 (6) 6330-6340 . DOI: 10.1021/acsnano.9b01015
This work is highlighted by Prof. Serge Lemay in the Perspective: “Nucleation of Electrochemically Generated Nanobubbles.“Lemay, ACS Nano 2019, 13, 6141-6144.
1. Zhao, X.; Ren, H.; Luo, L.*, “Gas Bubbles in Electrochemical Gas Evolution Reactions.” Langmuir 2019 35 (16), 5392-5408. DOI: 10.1021/acs.langmuir.9b00119
Other Publications:
2020
23. Robinson, D. A.; Edwards, M. A.; Liu, Y.; Ren, H.; White, H. S., Effect of Viscosity on the Collision Dynamics and Oxidation of Individual Ag Nanoparticles. J. Phys. Chem. C 2020, 124 (16), 9068–9076. DOI: 10.1021/acs.jpcc.0c01447
22. Ren, H.; Edwards, M. A.*; Wang, Y.; Gordon; White, H. S.*, “Electrochemically Controlled Nucleation of Single CO2 Nanobubbles Via Formate Oxidation at Pt Nanoelectrodes”. J. Phys. Chem. Lett. 2020, 11 (4), 1291-1296. DOI: 10.1021/acs.jpclett.9b03898
21. Qiu, Y.; Ren, H.; Edwards, M. A.; Gao, R.; Barman, K.; White, H. S., Electrochemical Generation of Individual Nanobubbles Comprising H2, D2, and HD. Langmuir 2020, 36 (22), 6073–6078. DOI: 10.1021/acs.langmuir.0c00232
20. Zhang, Y.; Robinson, D.; McKelvey, K.; Ren, H.; White, H. S.; Edwards, M. A. “A High-Pressure System for Studying Oxygen Reduction During Pt Nanoparticle Collisions” J. Electrochem. Soc. 2020, 167, 166507 DOI: 10.1149/1945-7111/abcde2
2019
19. Terry Weatherly, C. K.; Ren, H.; Edwards, M. A.; Wang, L.; White, H. S., Coupled Electron- and Phase-Transfer Reactions at a Three-Phase Interface. J. Am. Chem. Soc. 2019, 141 (45), 18091-18098. DOI: 10.1021/jacs.9b07283
18. Hunt, A. P.; Batka, A. E.; Hosseinzadeh, M.; Gregory, J. D.; Haque, H. K.; Ren, H.; Meyerhoff, M. E.; Lehnert, N., Nitric Oxide Generation on Demand for Biomedical Applications via Electrocatalytic Nitrite Reduction by Copper BMPA- and BEPA-Carboxylate Complexes. ACS Catal. 2019, . DOI: 10.1021/acscatal.9b01520
17. McCabe, M. M.; Hala, P.; Rojas-Pena, A.; Lautner-Csorba, O.; Major, T. C.; Ren, H.; Bartlett, R. H.; Brisbois, E. J.; Meyerhoff, M. E., “Enhancing Analytical Accuracy of Intravascular Electrochemical Oxygen Sensors via Nitric Oxide Release Using S-Nitroso-N-Acetyl-Penicillamine (SNAP) Impregnated Catheter Tubing.” Talanta 2019 205, 120077. DOI: 10.1016/j.talanta.2019.06.077
2018
16. Tan, C.; Fleming, A. M.; Ren, H.; Burrows, C. J.; White, H. S., “γ-Hemolysin Nanopore is Sensitive to Guanine-to-Inosine Substitutions in Double-Stranded DNA at the Single-Molecule Level.” J. Am. Chem. Soc. 2018 140 (43), 14224–14234 DOI: 10.1021/jacs.8b08153
15. Edwards, M. A.; Robinson, D. A.; Ren, H.; Cheyne, C. G.; Tan, C. S.; White, H. S. “Nanoscale Electrochemical Kinetics & Dynamics: The Challenges and Opportunities of Single-Entity Measurements.” Faraday Discuss. 2018 210 (0), 9-28. DOI: 10.1039/C8FD00134K
14. Robinson, D. A.; Edwards, M. A.; Ren, H.; White, H. S., “Effects of Instrumental Filters on Electrochemical Measurement of Single-Nanoparticle Collision Dynamics.” ChemElectroChem 2018, 5, 3059. DOI: 10.1002/celc.201800696
13. Soto, A.; German, S. R.; Edwards, M. A.; Ren, H.; White, H. S.,“The Nucleation Rate of Single O2 Nanobubbles at Pt Nanoelectrodes.” Langmuir 2018, 34 (25), 7309-7318. DOI: 10.1021/acs.langmuir.8b01372
12. Ren, H.; Cheyne, C.; Fleming, A. M.; Burrows, C. J.; White, H. S., “Titration of a Single Captured Molecule in a Protein Nanoreactor Reveals Protonation/Deprotonation Mechanism of a C:C Mismatch in Nano-Confinement.” J. Am. Chem. Soc. 2018, 140 (15), 5153–5160. DOI: 10.1021/jacs.8b00593
11. Zeng, T.; Fleming, A. M.; Ding, Y.; Ren, H.; White, H. S.; Burrows, C. J., “Nanopore Analysis of the 5-Guanidinohydantoin to Iminoallantoin Isomerization in Duplex DNA” J. Org. Chem 2018, 83 (7), 3973-3978. DOI: 10.1021/acs.joc.8b00317
10. German, S. R.; Edwards M. A.; Ren, H.; White, H. S.,“Critical Nuclei Size, Rate, and Activation Energy of H2 Gas Nucleation.” J. Am. Chem. Soc. 2018 140 (11), 4047–4053. DOI: 10.1021/jacs.7b13457
2017
9. Yu, Q.; Zajda, J.; Brisbois, E. J.; Ren, H, Toomasian, J.; Rojas-Pena, A.; Bartlett, R. H.; Hunt, A.; Lehnert, N.; Meyerhoff, M. E. et. al., “Portable Nitric Oxide (NO) Generator Based on Electrochemical Reduction of Nitrite for Potential Applications in Inhaled NO Therapy and Cardiopulmonary Bypass Surgery.” Mol. Pharmaceutics 2017, 14 (11), 3762–3771. DOI:10.1021/acs.molpharmaceut.7b00514
8. Ren, H.; German, S. R.; Edwards, M. A.; Chen, Q.; White, H. S., “Electrochemical Generation of Individual O2 Nanobubbles via H2O2 Oxidation.” J. Phys. Chem. Lett. 2017, 8 (11), 2450–2454. DOI: 10.1021/acs.jpclett.7b00882
2016
7. Ren, H.; Bull, J. L.; Meyerhoff, M. E., “Transport of Nitric Oxide (NO) in Various Biomedical Grade Polyurethanes: Measurements and Modeling Impact on NO Release Properties of Medical Devices.” ACS Biomater. Sci. Eng. 2016, 2 (9), 1483–1492. DOI: 10.1021/acs.jpclett.7b00882
6. Ren, H.; Wu, J.; Colletta, A.; Meyerhoff, M. E.; Xi, C., “Efficient Eradication of Mature Pseudomonas Aeruginosa Biofilm via Controlled Delivery of Nitric Oxide Combined with Antimicrobial Peptide and Antibiotics. Frontiers in Microbiology, 2016, 7 (1260). DOI: 10.3389/fmicb.2016.01260
5. Lee, W. §; Ren, H.§; Wu, J; Xi, C.; Meyerhoff, M. E., “Electrochemically Modulated Nitric Oxide Release from Flexible Silicone Rubber Patch: Antimicrobial Activity for Potential Wound Healing Applications.” ACS Biomater. Sci. Eng. 2016, 2 (9), 1432–1435. DOI: 10.1021/acsbiomaterials.6b00360
2015
4. Ren, H.; Coughlin, M. A.; Major, T. C.; Aiello, S.; Rojas, A. P.; Bartlett, R. H.; Meyerhoff, M. E. “Improved In Vivo Performance of Amperometric Oxygen (PO2) Sensing Catheters via Electrochemical Nitric Oxide Generation/Release.” Anal. Chem. 2015, 87 (16), 8867–8872. DOI: 10.1021/acs.analchem.5b01590
3. Zheng, Z.; Ren, H.; Meyerhoff, M. E., “Highly Sensitive Amperometric Pt-Nafion Gas Phase Nitric Oxide (NO) Sensors: Performance and Application in Characterizing NO-Releasing Biomaterials.” Anal. Chim. Acta 2015, 887 (16), 186–191. DOI: 10.1016/j.aca.2015.06.016
2. Ren, H.; Colletta, A.; Koley, D.; Wu, J.; Xi, C.; Major, T.; Bartlett, R. H; Meyerhoff, M. E., “Thromboresistant/Anti-Biofilm Catheters via Electrochemically Modulated Nitric Oxide Release.” Bioelectrochemistry 2015, 104, 10–16. DOI: 10.1016/j.bioelechem.2014.12.003
2014
1. Ren, H.; Wu, J.; Xi, C.; Lehnert, N.; Major, T.; Bartlett, R. H.; Meyerhoff, M. E., “Electrochemically Modulated Nitric Oxide (NO) Releasing Biomedical Devices via Copper(II)-Tri(2-pyridylmethyl)amine Mediated Reduction of Nitrite.” ACS Appl. Mater. Interfaces 2014, 6 (6), 3779–3783. DOI: 10.1021/am406066a
Patent Applications:
1. “Gas Delivery Devices”, US Patent, (US2017021655)
2. “Nitric Oxide Delivery Devices”, US Patent, (US 20140294672)
3. “Nitric Oxide Generation Formulations and Kits”, US Patent, (PCT/US2017/019136)



