Research Interest:
Correlative Electrochemical Imaging for Electrocatalysis
Electrochemical Delivery for Biology
We are interested in nanoelectrochemistry and single-entity electrochemistry. We use scanning electrochemical probe microscopy to study fundamental electrocatalytic processes for energy storage and conversion at the nanoscale to reveal their heterogeneity and stochasticity. We are also devoted to developing electrochemical sensors and on-demand drug release devices for biomedical applications.
Tools for Nanoelectrochemistry
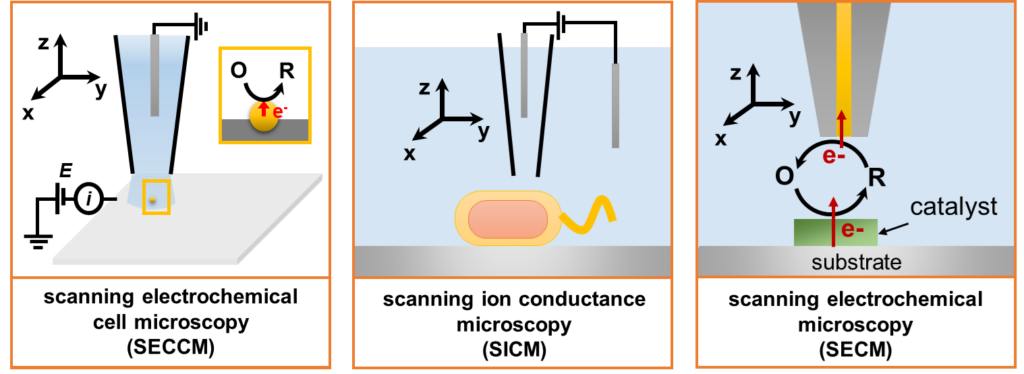
Nanoscale scanning electrochemical probe microscopy tools, including SECCM, SICM, and SECM, will be used to reveal the heterogeneity of electrochemical processes, including electrocatalysis, at the nanoscale.
- Blount, B.; Juarez, G.; Wang, Y.; Ren, H.*, iR Drop in Scanning Electrochemical Cell Microscopy. Faraday Discuss., 2022, 233, 149 – 162. DOI: 10.1039/D1FD00046B
- Wang, Y.; Li, M.; Ren, H., Voltammetric Mapping of Hydrogen Evolution Reaction on Pt Locally via Scanning Electrochemical Cell Microscopy. ACS Measurement Science Au, 2022 Accepted. DOI: 10.1021/acsmeasuresciau.2c00012
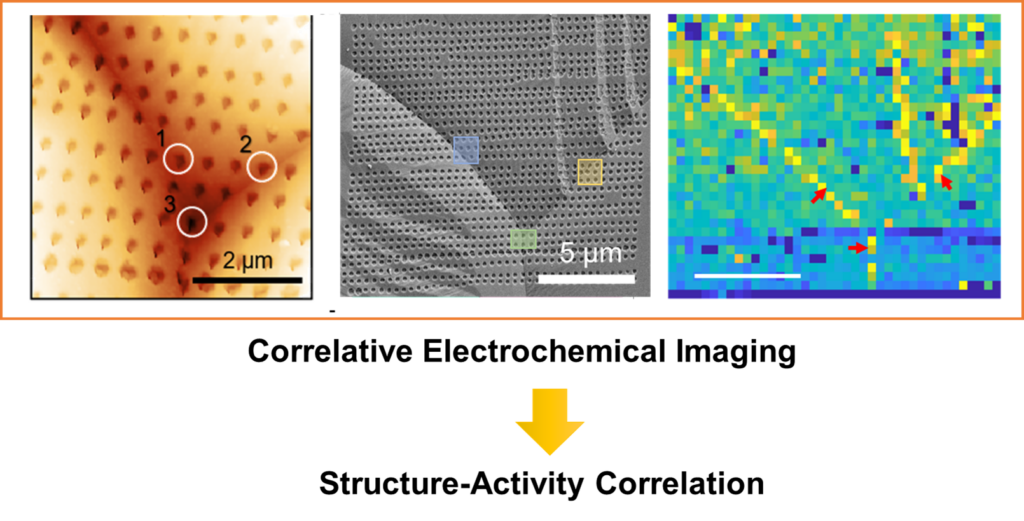
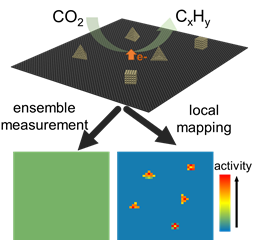
Structure-Activity Correlation in Electrocatalysis at Single Sites
We use colocalized imaging methods, including SECCM, AFM, and TEM, to characterize the activity and structure of electrochemical interfaces. In this way, we will not only reveal the heterogeneity in the active sites in electrocatalysis but also elucidate their structural origin.
Relevant papers:
- Li, M.; Ye, K.-H.; Qiu, W.; Wang, Y.; Ren, H.*, Heterogeneity between and within Single Hematite Nanorods as Electrocatalysts for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2022, 144 (12), 5247–5252. DOI: 10.1021/jacs.2c00506
- Zheng, H.; Li, M.; Chen, J.; Quan, A.; Ye, K.; Ren, H.; Hu, S.; Cao, Y., Strain tuned efficient heterostructure photoelectrodes. Chin. Chem. Lett. 2022, 33 (3), 1450-1454. DOI:10.1016/j.cclet.2021.08.062
Initiation of Corrosion

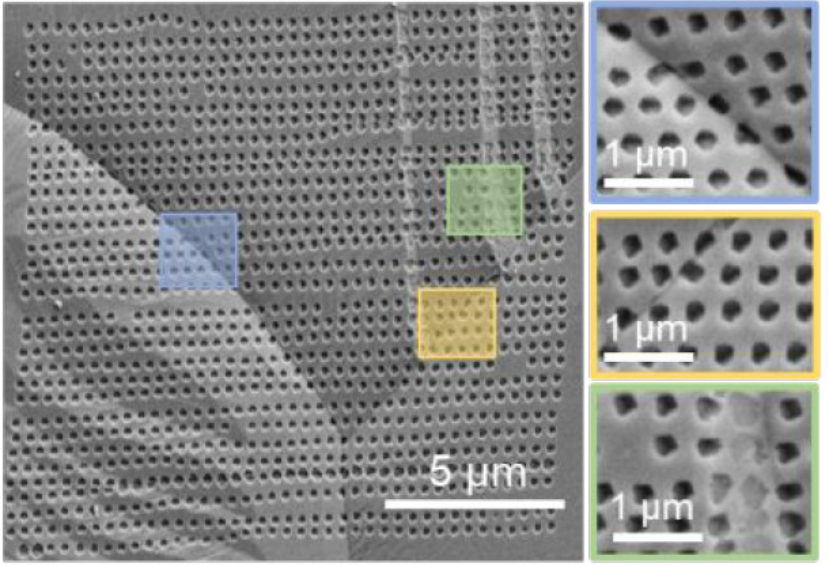
Image Credit: Corrosion | Scuddr | Flickr
Corrosion is an old but unsolved problem with an annual global cost of $2.5 trillion. Corrosion is an electrochemical process in nature (i.e., galvanic cell), and corrosion initiates at the nanoscale. We used correlative imaging to study the initiation of corrosion, map the local kinetics of metal dissolution, and reveal the structural nature of sites for corrosion initiation.
Relevant papers:
- Wang, Y; Li, M.; Gordon, E.; Ye, Z.; Ren, H.*, Nanoscale Colocalized Electrochemical and Structural Mapping of Metal Dissolution Reaction, Anal. Chem. 2022, 94, 25, 9058–9064. DOI: 10.1021/acs.analchem.2c01283
- Li, M.; Wang, Y.; Blount, B.; Ren, H.*, Stochastic Local Breakdown of Oxide Film on Ni from Identical-Location Imaging: One Single Site at a Time, Nano Letters, 2022, 22 (15) 6313–6319. DOI:10.1021/acs.nanolett.2c02018
Precision Delivery for Biology
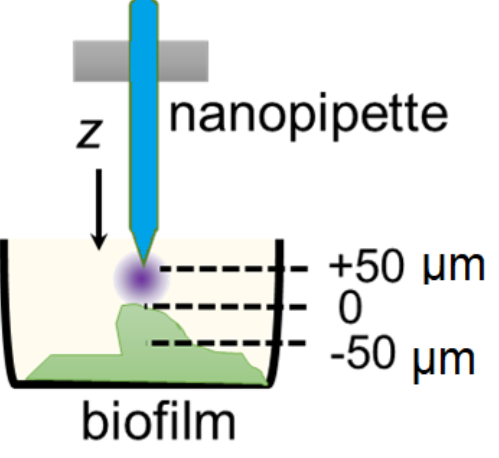
We will develop nanoscale electrochemical tools to precisely deliver molecules for biological studies with high spatial and temporal resolution and ultimately for single-cell studies.
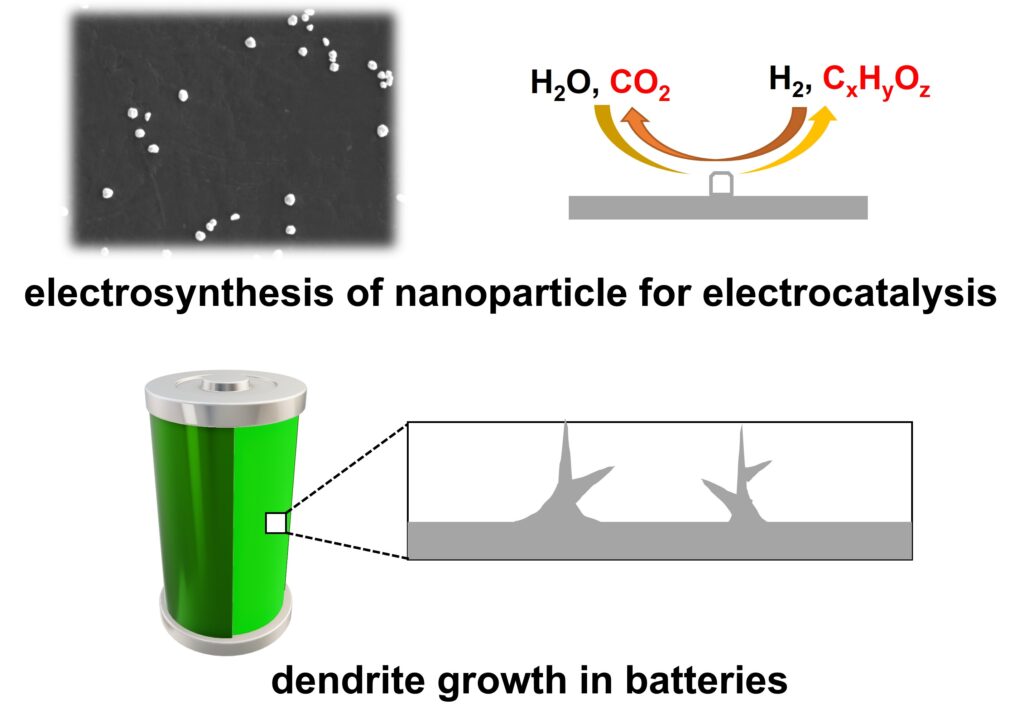
Electrochemical Phase Transition
New phases are often formed during electrochemical processes, e.g., during battery charging. Controlling the nucleation and growth and the solid is important, from preventing the dendrite formation for batteries of better performance to the synthesis of nanoparticles for better electrocatalysis.
The formation of a new phase often needs a process called nucleation. Classical nucleation theory (CNT) suggests that there is a minimum dimension of the new phase that needs to be formed before the growth of the new phase is energetically favorable, as shown in the Figure above. However, the nucleation events are often stochastic and heterogeneous over the electrode surface.
To address this issue, we will use nanoelectrochemical techniques to probe the nucleation process and its dependence on the substrate structure.
Relevant papers:
Edwards, M. A.*; White, H. S.; Ren, H.*, “Voltammetric Determination of the Stochastic Formation Rate and Geometry of Individual H2, N2, and O2 Bubble Nuclei.” ACS Nano 2019 13 (6) 6330-6340 . DOI: 10.1021/acsnano.9b01015
Wang, Y.; Gordon, E.; Ren, H.*, “Mapping the Nucleation of H2 Bubbles on Polycrystalline Pt via Scanning Electrochemical Cell Microscopy”. J. Phys. Chem. Lett. 2019, 3887-3892. DOI: 10.1021/acs.jpclett.9b01414
Blount, B.; Kilner, K.; Hu, H.; Gohmann, D.; Gordon, E.; Wang, Y.; Ren, H.*, ”Electrochemically Induced Nucleation of a Nanoscopic Ionic Solid”. J. Phys. Chem. C. 2020 124 (31), 17413-17417. DOI: 10.1021/acs.jpcc.0c05009
Heterogeneity of the Electrical Double Layer
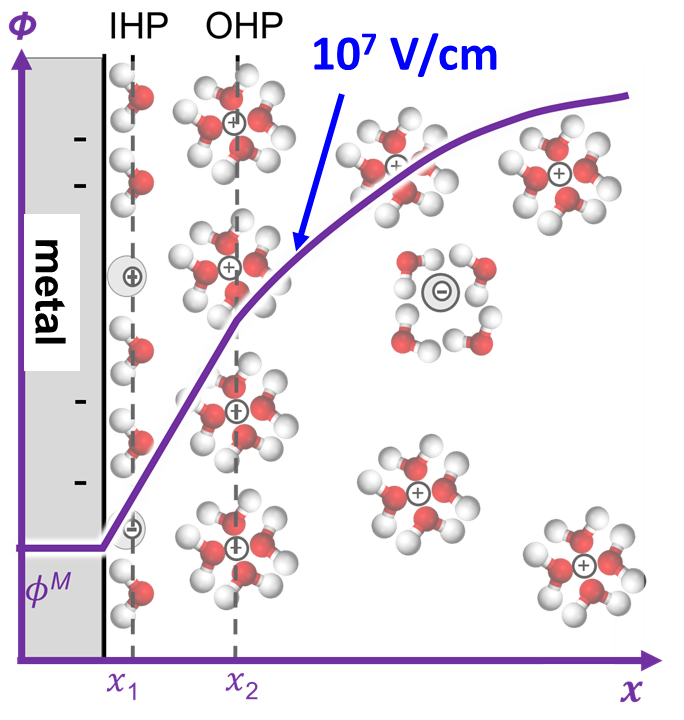
Electrical double layer (EDL) is ubiquitous in electrochemistry and is fundamentally important for the electrode/electrolyte interface. We are developing nanoscale techniques to probe the EDL structure and its dependence on the electrode structure. We have shown the measurement of the local potential of zero charge (PZC) at Pt and Au electrodes and found the PZC value is dependent on the crystal orientation.
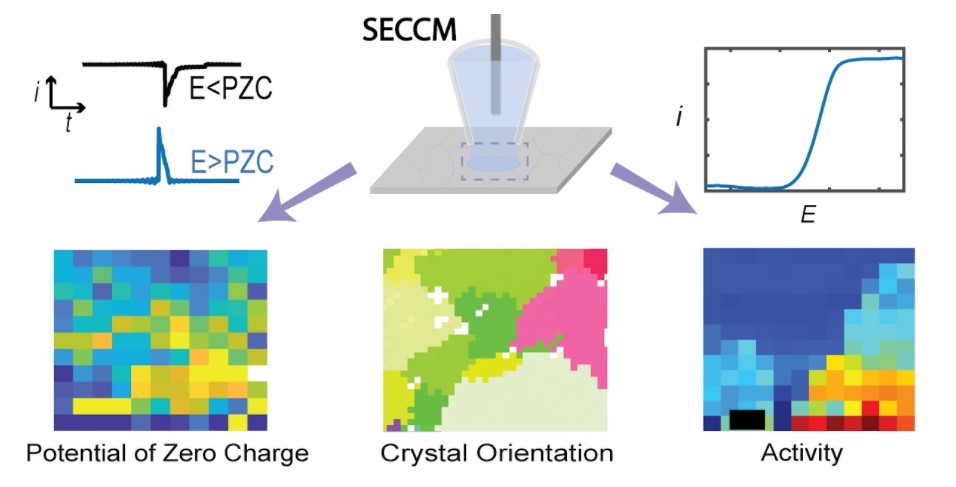
Relevant papers:
- Wang, Y.; Gordon, E.; Ren, H.*, “Mapping the Potential of Zero Charge (PZC) and Electrocatalytic Activity of Metal-Electrolyte Interface via a Grain-by-Grain Approach.” Anal. Chem. 2020, 92 (3), 2859-2865. DOI: 10.1021/acs.analchem.9b05502
- Terry Weatherly, C. K.; Ren, H.; Edwards, M. A.; Wang, L.; White, H. S., Coupled Electron- and Phase-Transfer Reactions at a Three-Phase Interface. J. Am. Chem. Soc. 2019, 141 (45), 18091-18098. DOI: 10.1021/jacs.9b07283
Funding:
We gratefully acknowledge the generous support from the following organizations:

CHE-2240113: CAREER: Developing Nanoscale Scanning Electrochemical Probe Systems to Reveal the Electrical Double Layer at Electrocatalytic Interfaces

1R35GM147172: R35- NIH Maximizing Investigators’ Research Award (MIRA): “Spatially and temporarily resolved precision delivery for quantitative biological studies”

F-2158-20230405: Welch Research Grant

SciaLog: Negative Emission Science (NES) Award

W911NF-20-1-0304: DARPA Young Faculty Award (YFA
DARPA Director’s Award

61155-DNI5: ACS Petroleum Research Fund, Doctoral New Investigator (DNI) Grant


